Le Chatelier's principle
Le Chatelier's principle states
that when any change is made to the conditions of an equilibrium the position of
the equilibrium moves in the direction that minimises the change. Some
reactions are reversible in dynamic equilibrium the rate of forward (right)
reaction is the same as the backward (left) reaction concentrations of products
and reactants stay the same and dynamic equilibrium can only happen in a closed
system Can be any combination of reactants and products, doesn’t have to be
half and half and effects of changes in conditions predicted by Le Chatelier’s
principle.
“If a system at equilibrium is disturbed, the
equilibrium moves in the direction that tends to reduce that disturbance”
Rate of forward or backward reaction increases to minimise
change both forward and reverse rates are speeded up equally and No effect on
the yield . We get no more product, we just get it faster. Industrial equilibrium
use compromise conditions that balance yield against reaction rate, cost and
risks of equipment needed for conditions, side reactions and catalysts. Ammonia,
ethanol and methanol are all produced industrially using a compromise
temperature and pressure
The table below shows some of the properties that occur when
Le chatelier's principle is in use:
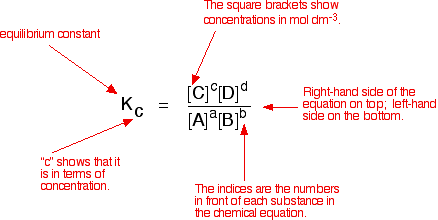
The equilibrium constant is the ratio of product concentration
to reactant concentration which is raised to its appropriate power. this only varies
for a reaction when the temperature is changed and it indicates whether the position
of equilibrium to the left or to the right.
The equilibrium constant formula is:
In industry a compromise may be needed between yield and rate
such as for exothermic reaction, a lower
temperate gives you a higher yield and a lower temperature saves costs in terms
of no need to pay for electricity to create heat or fuel on the other hand a lower
temperature slows down the forward backward reactions so it would take longer to
reach equilibrium.
**REMEMBER TO STAY POSITIVE LIKE A PROTON!!**